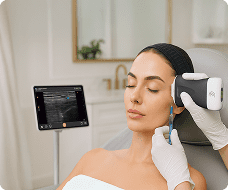
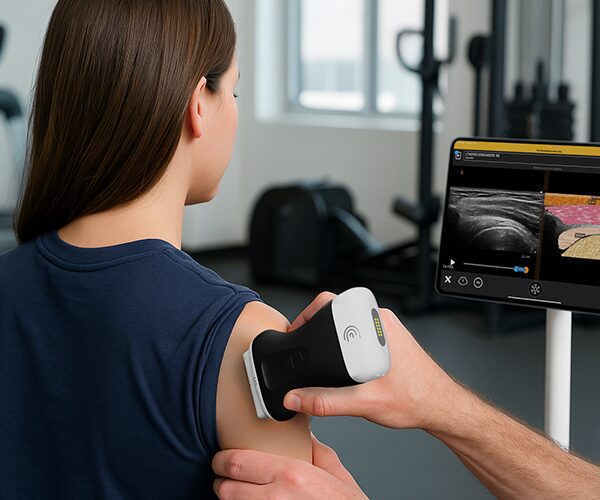
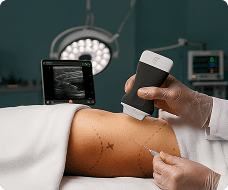
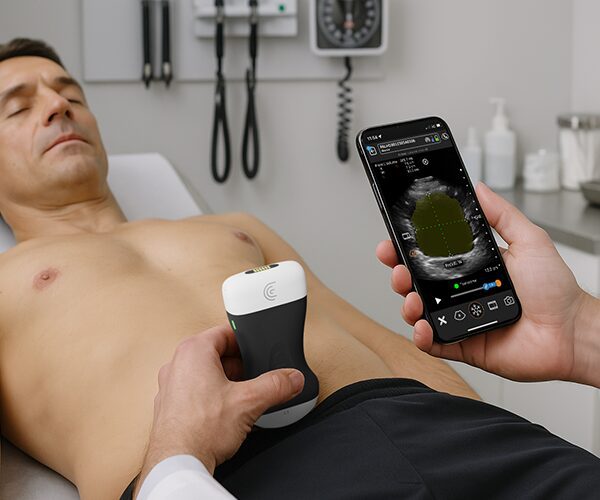
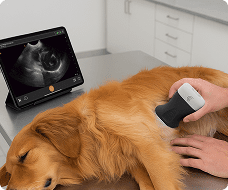
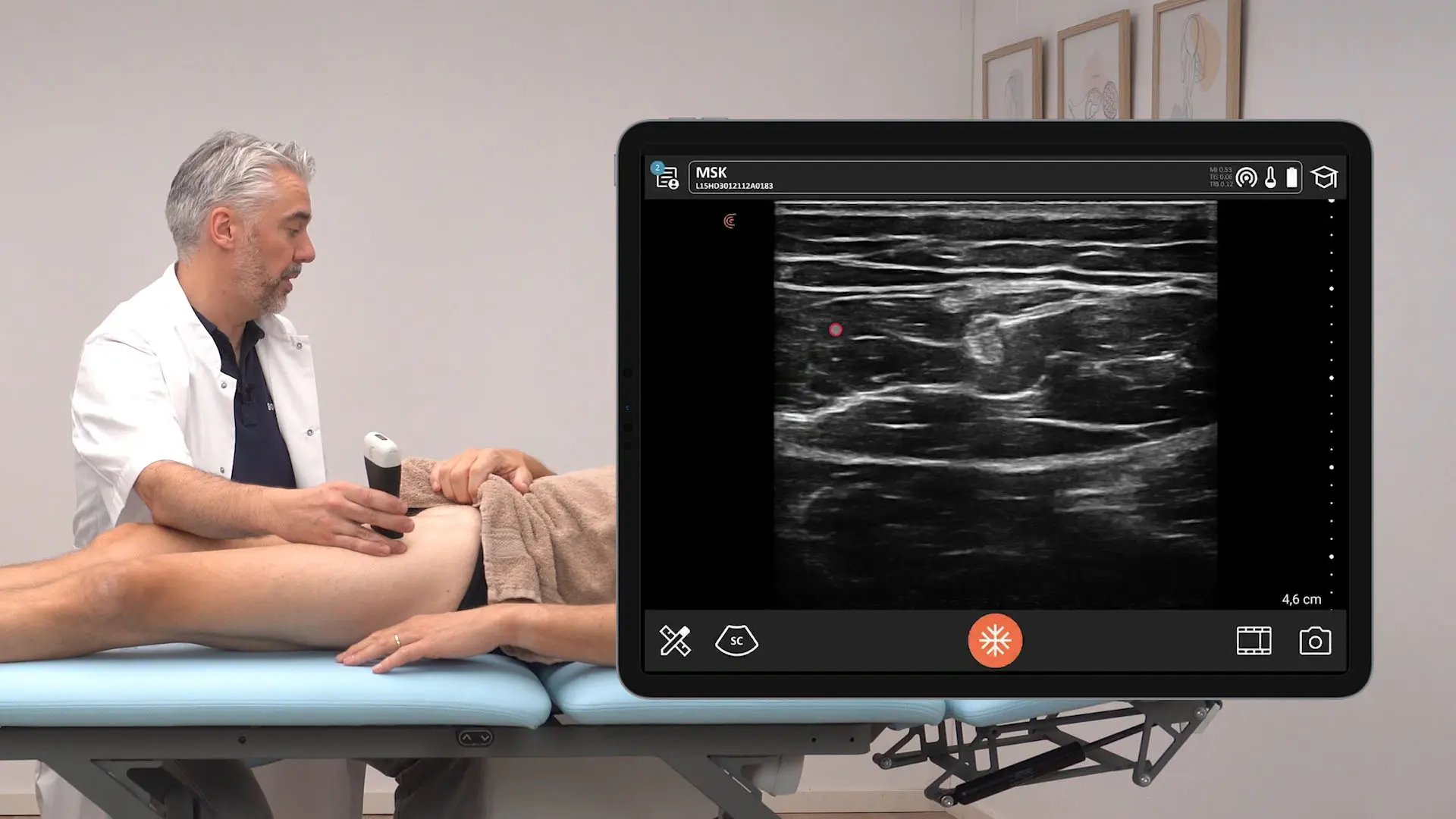
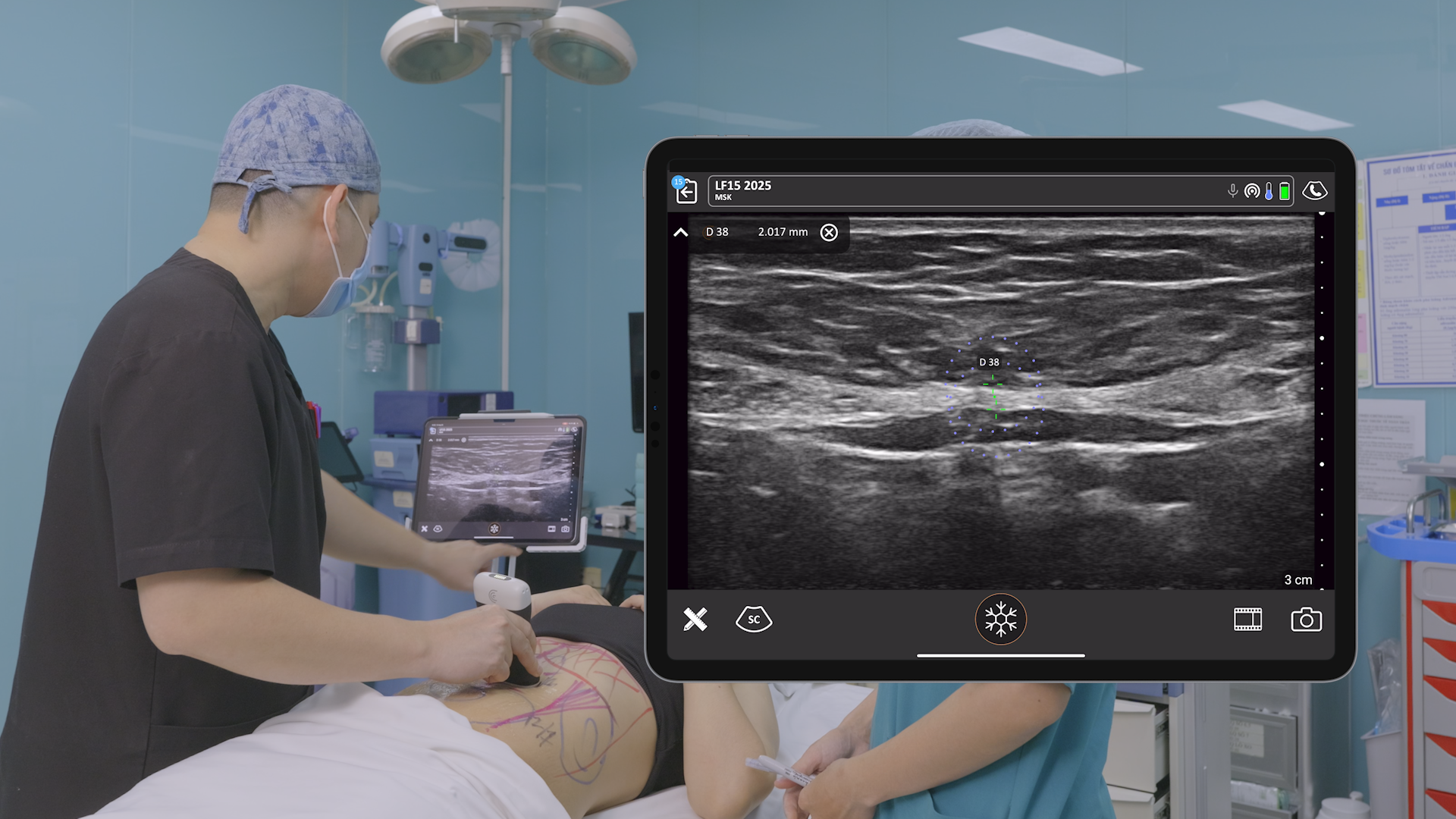
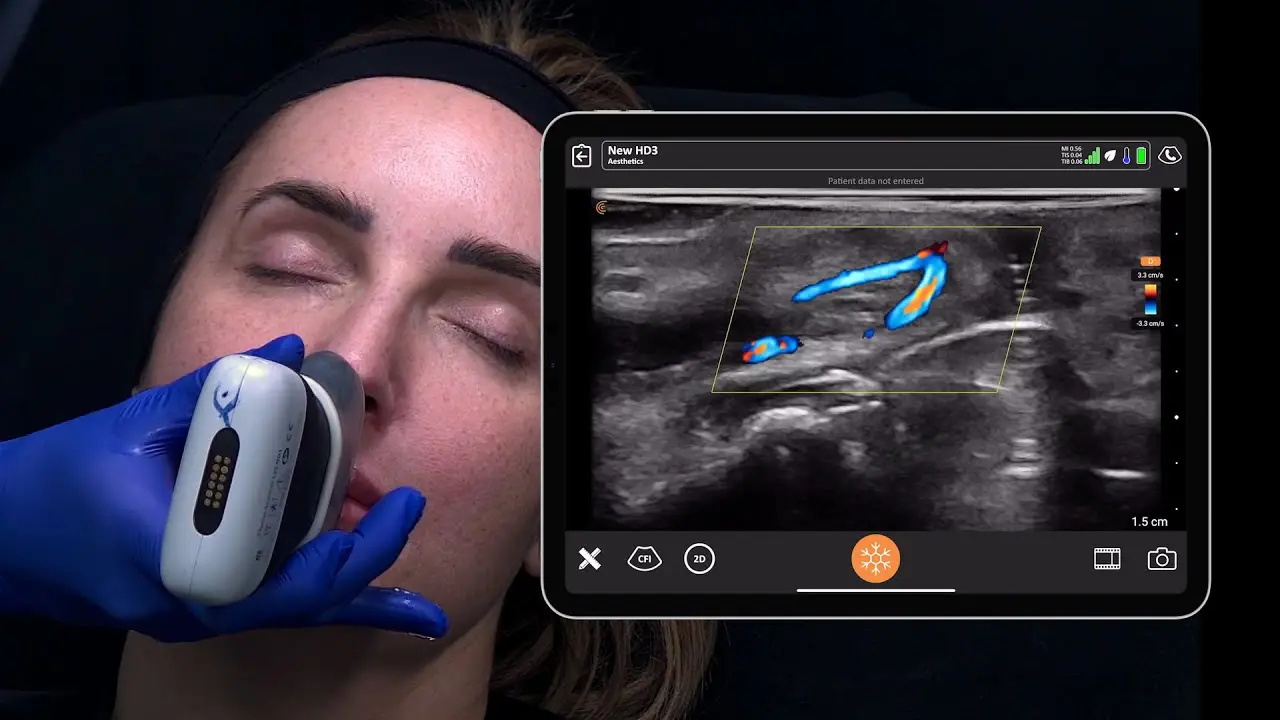
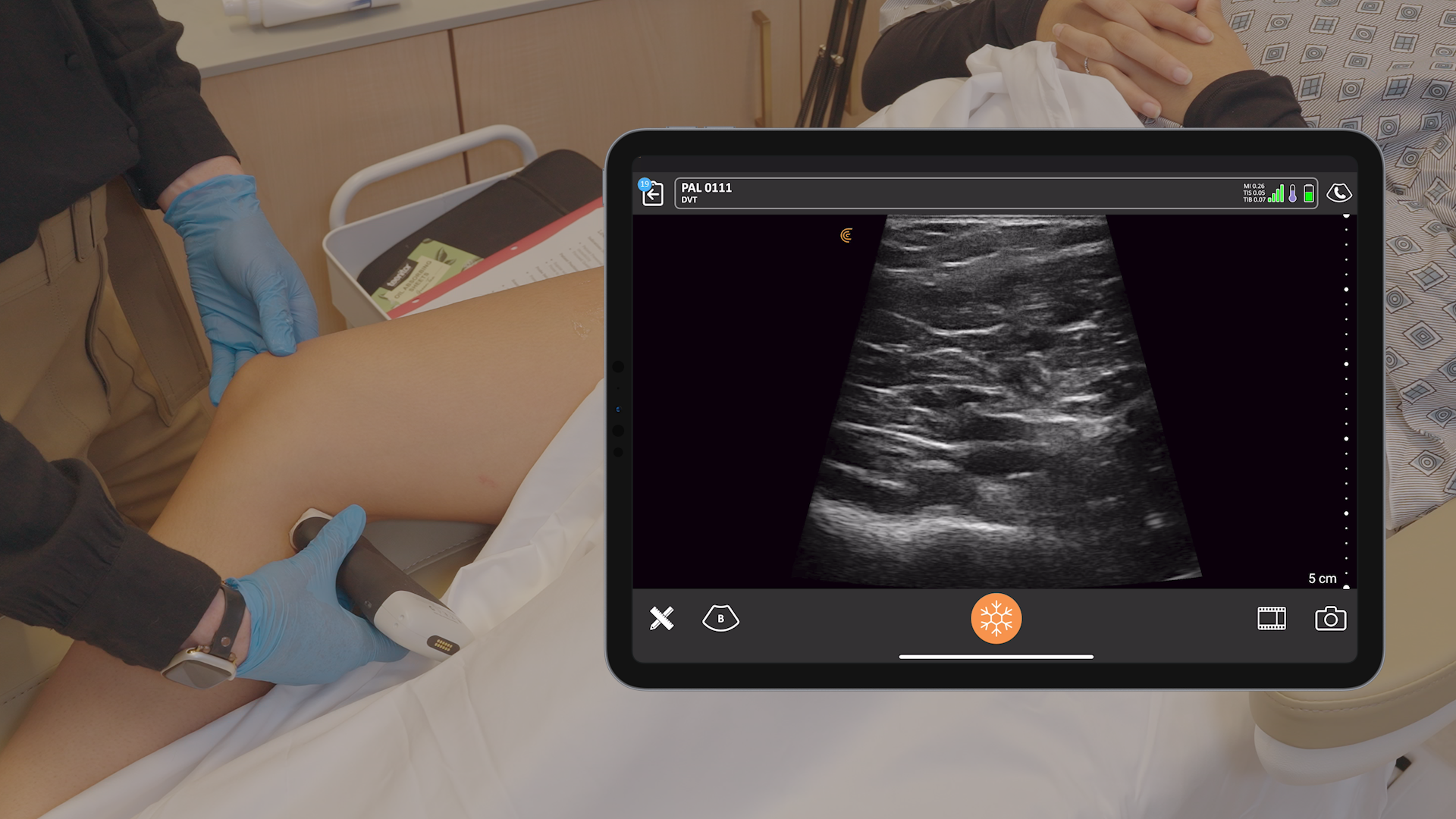
The U.S. Food and Drug Administration (FDA) has cleared the L7 and C3 Clarius Wireless Ultrasound Scanners for sale in the United States. Compatible with the latest Android and iOS smartphones and tablets, Clarius scanners are available now for medical professionals at www.clarius.me/shop.
Clarius is the future of patient care. The image quality is amazing for any scanner, much less one that fits in my pocket,” said Dr. Steven Steinhubl, Director of Digital Medicine at the Scripps Translational Science Institute. “The ability to wirelessly connect it to any Apple or Android device means that anyone on my team can use it with whatever they already carry around in their pocket.”
To ensure customer satisfaction, Clarius offers customers the option to return the product after a one-week trial period for a small handling fee. Clarius scanners are also available for order by telephone or the company’s network of sales partners across the country.
Read the full story here.