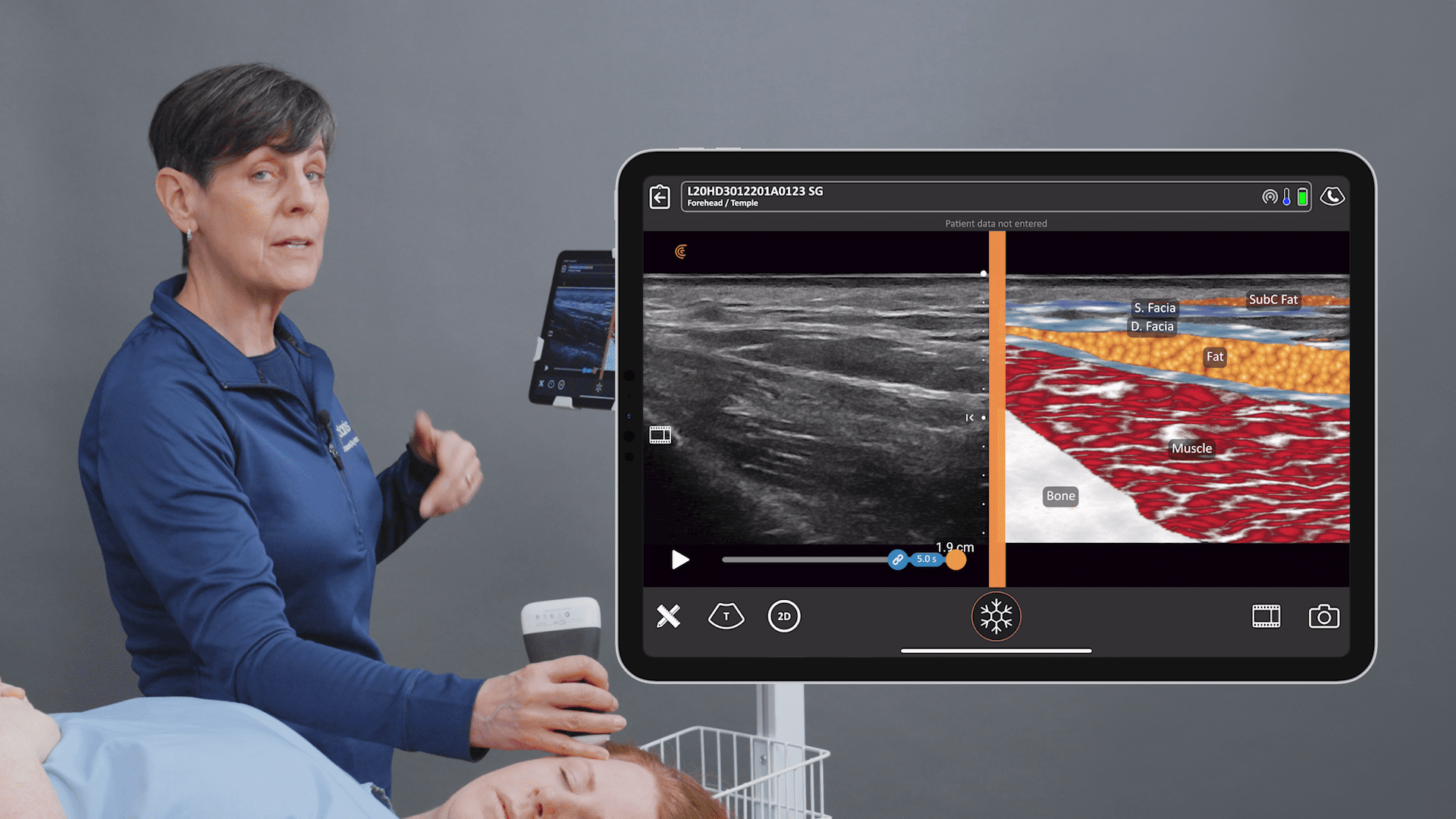
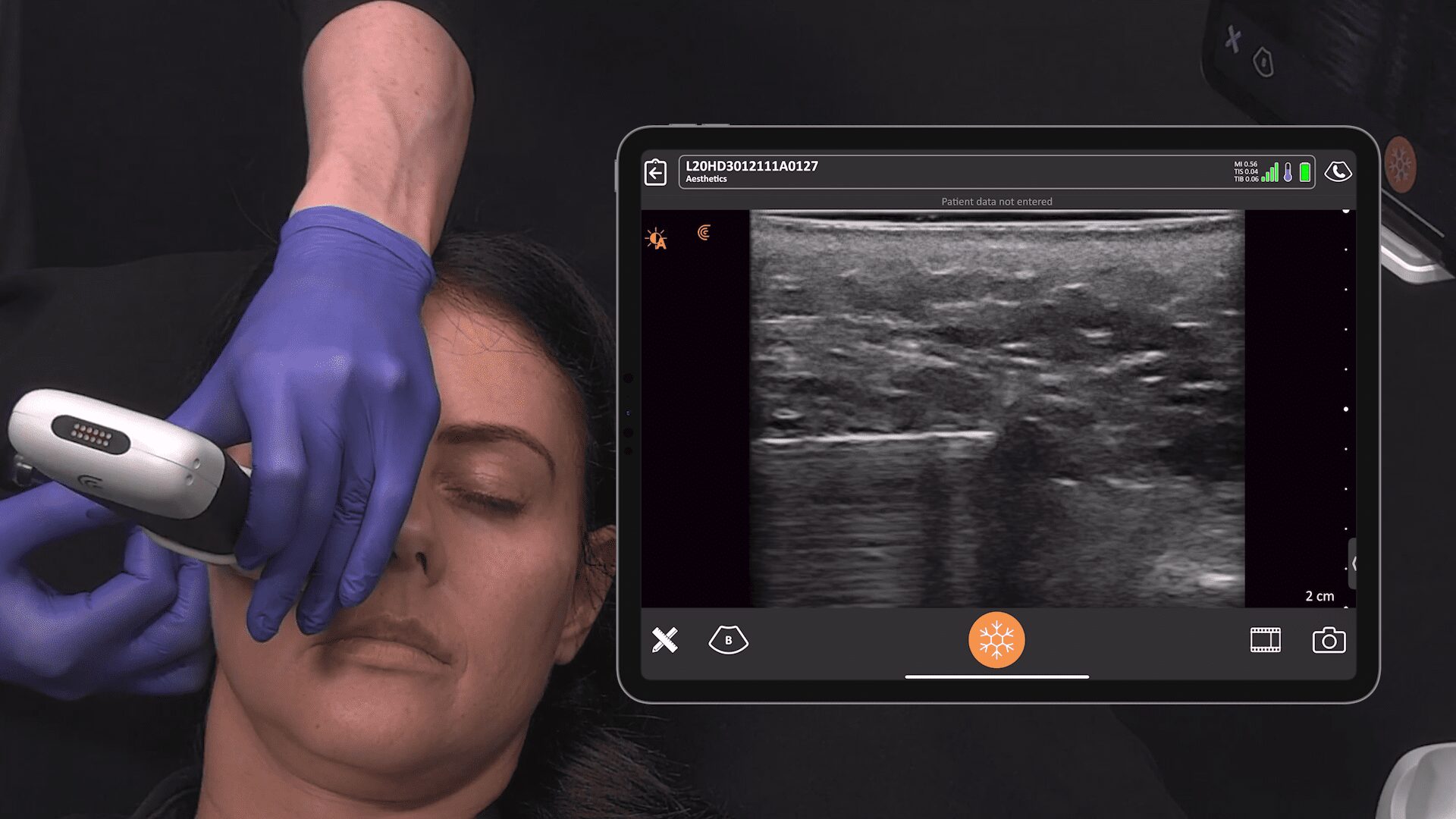
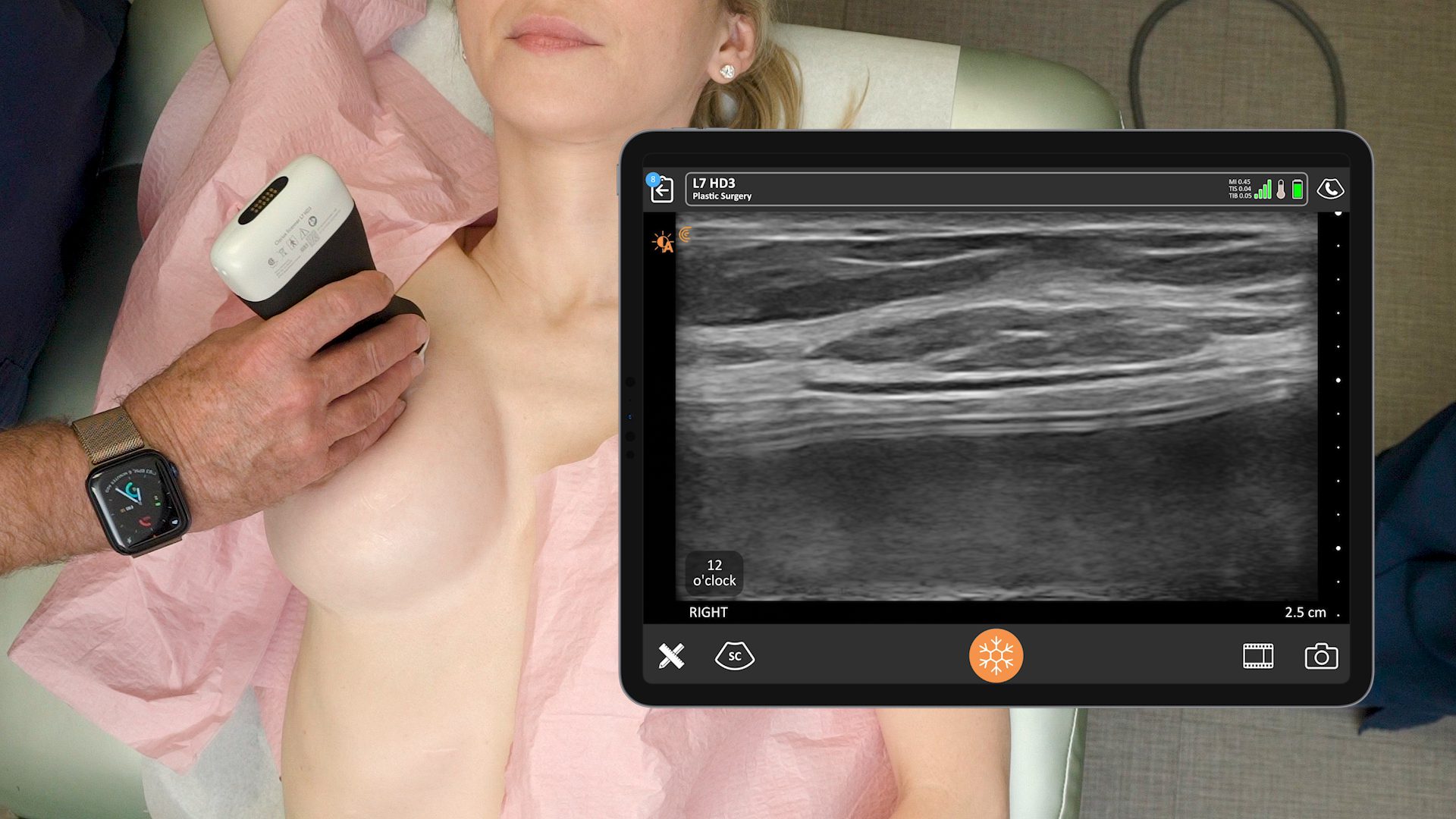
Historically, the U.S. Food and Drug Administration has recommended magnetic resonance imaging (MRIs) for silicone gel implants to screen for silent rupture at 5 to 6 years after implantation, and yet, less than 5% of patients have complied due to the high cost and inconvenience of screening. With the FDA adding ultrasound in recent years for screening breasts to detect silent ruptures, we are seeing increased interest in point-of-care ultrasound among plastic surgeons, who can now immediately scan concerned patients rather than sending them away for an MRI.
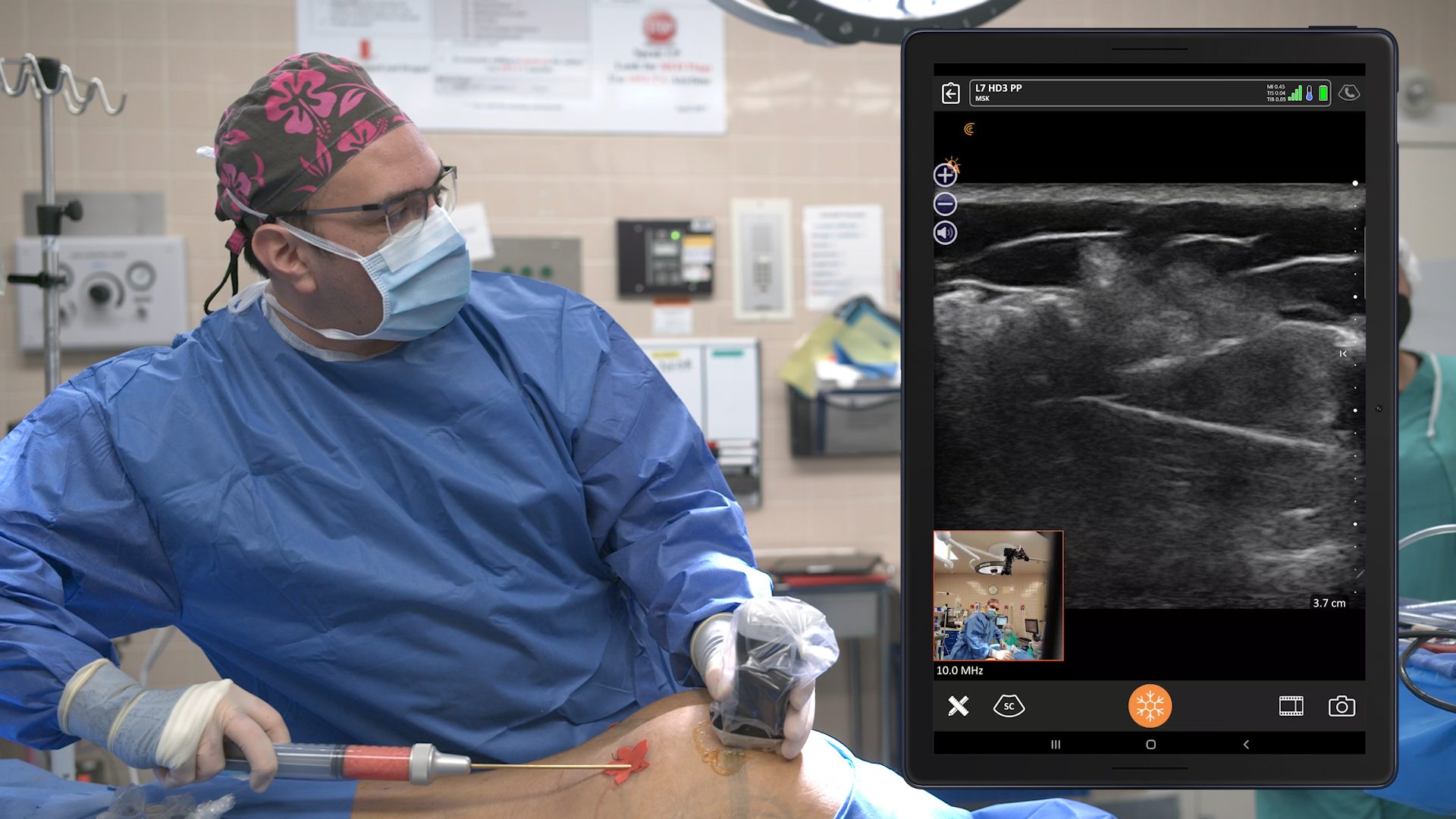
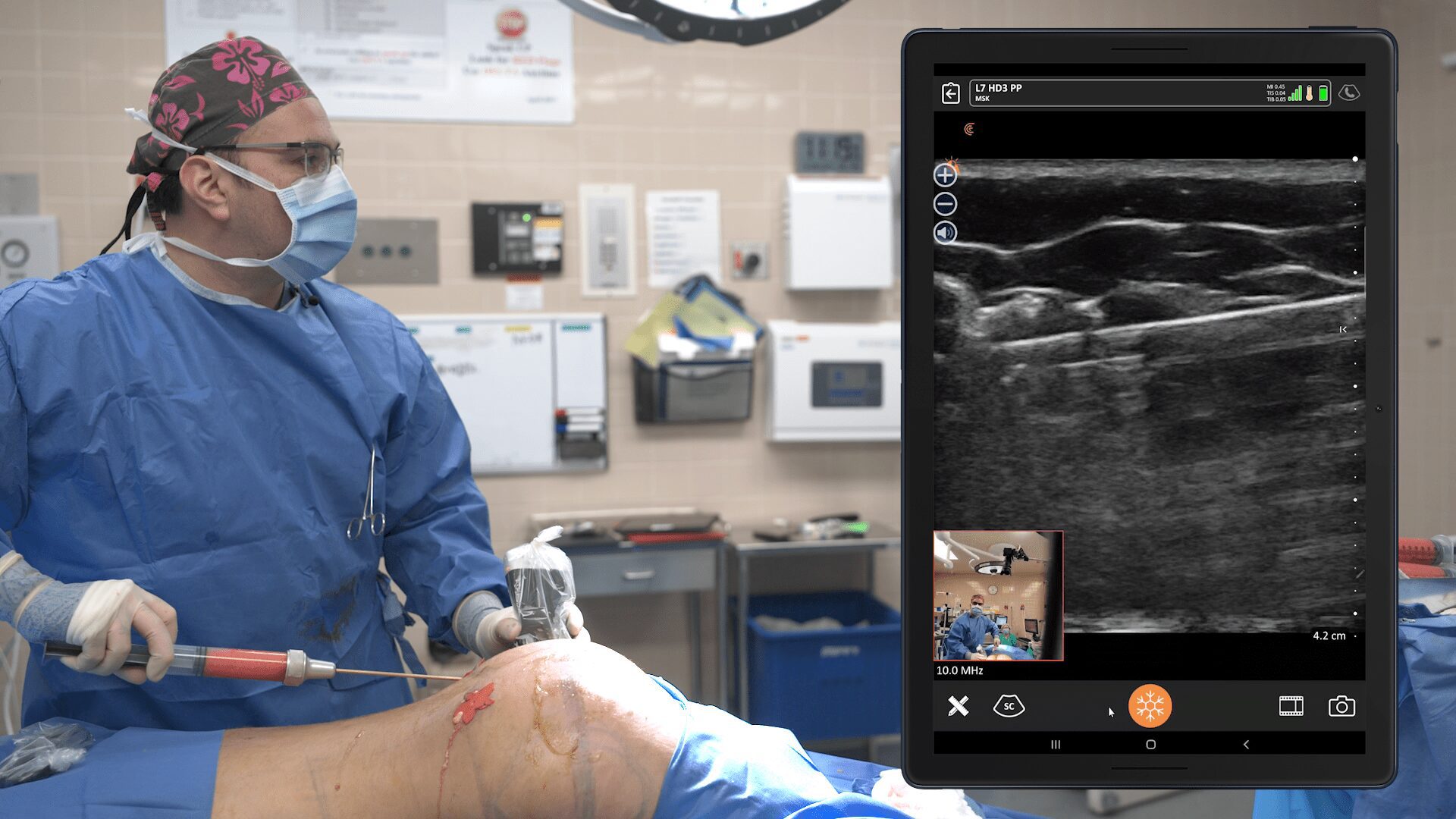
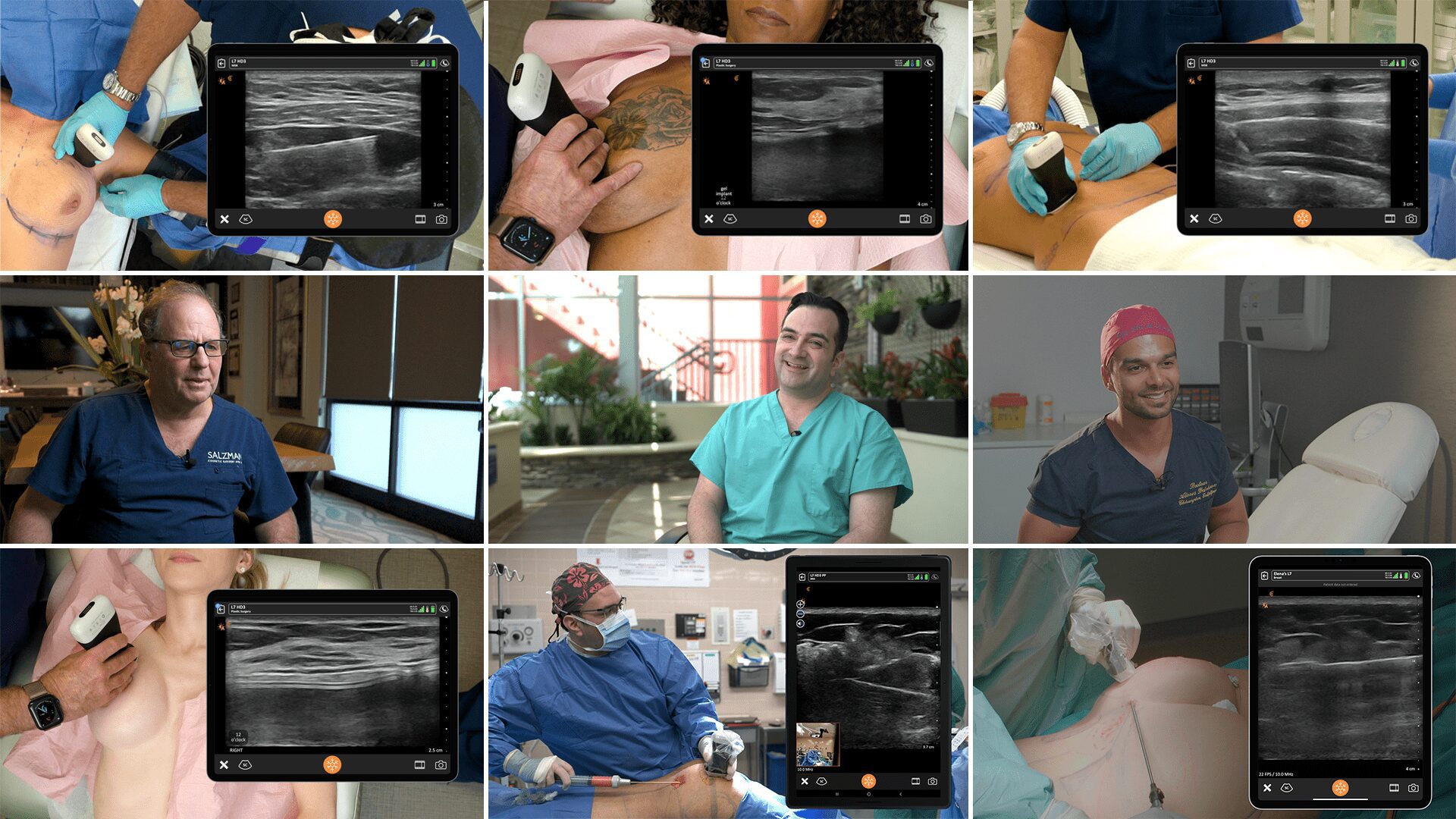
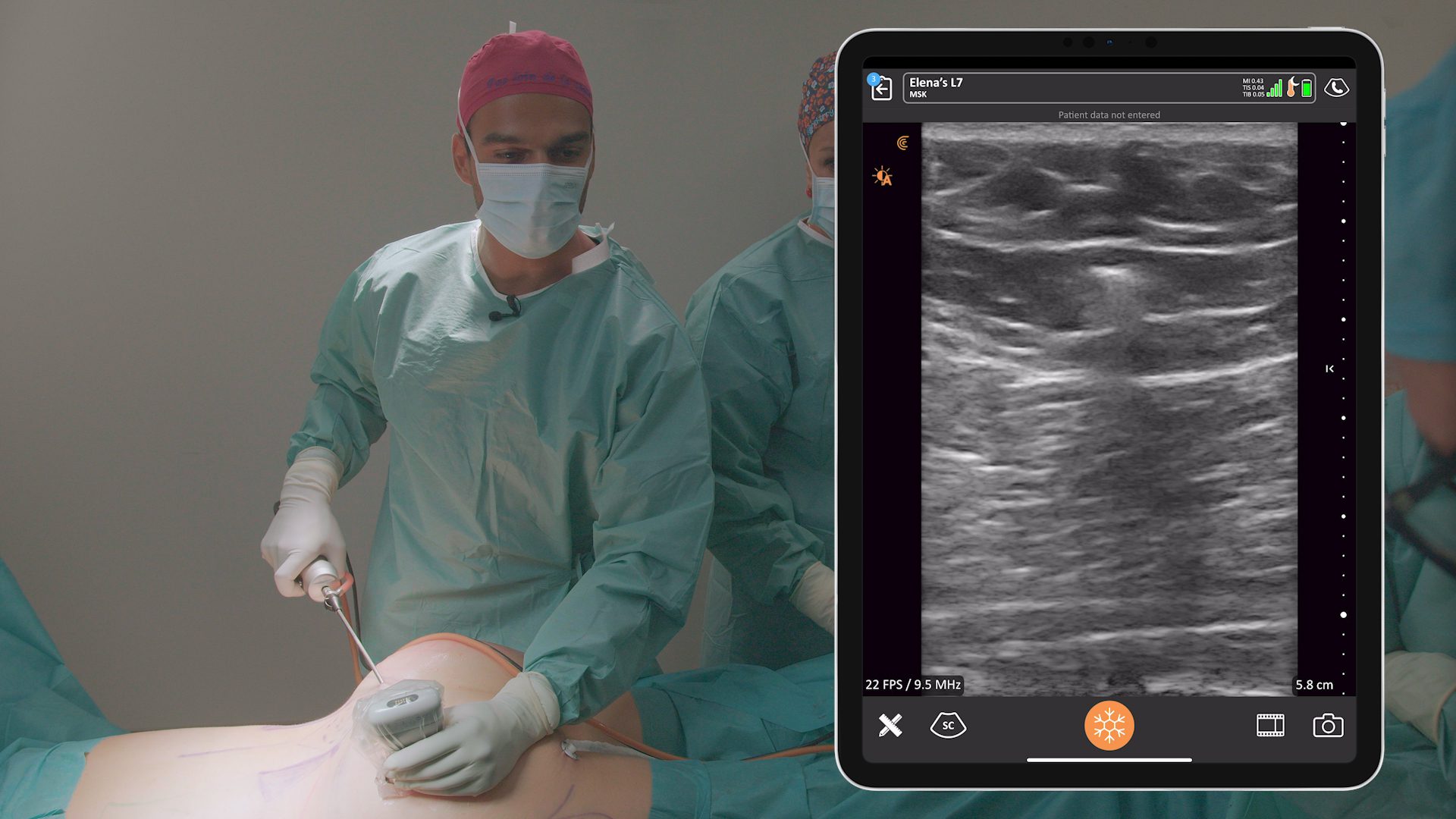
An early adopter and renowned expert, Dr. Marc Salzman is a plastic surgeon based in Kentucky who has been using ultrasound at his plastic surgery practice since 2012. Concerned about the lack of screening for ruptured implants, he has been spearheading a study that screened 584 women over the past two years using the Clarius L7 HD handheld ultrasound as a low-cost and convenient rapid screening method. His article about the study was recently published in the Plastic and Reconstructive Surgery Journal.
Highlights from Silent Rupture Study
Method
- Screenings took place at nine private practices where plastic surgeons, registered nurses, and certified medical assistants received 1-day training with the Clarius L7 handheld ultrasound and an iPad.
- Suspect scans conducted by less experienced screeners were reviewed by experienced plastic surgeons.
- Scan results were correlated with results from surgical findings.
- Women were surveyed about their feelings about silent ruptures.
Results
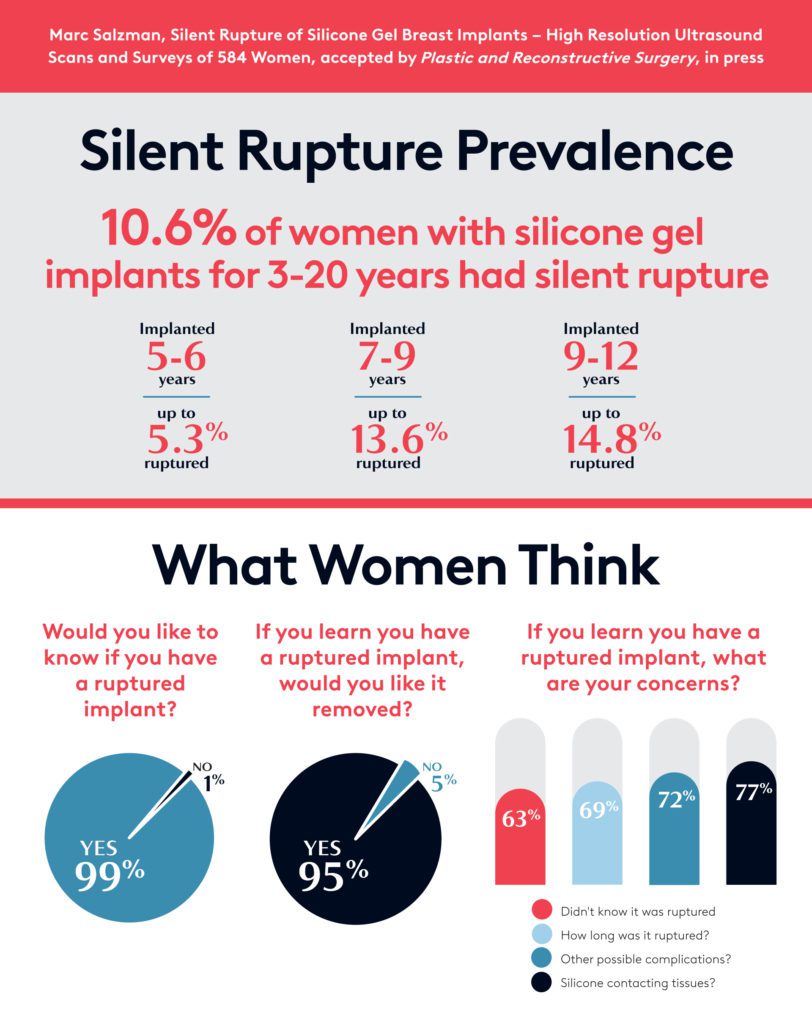
- The results indicated that women feel concerned about possible silent ruptures.
- 82 of the 584 women screened indicated implant ruptures (14%).
- Of the women who underwent surgery, 10.6% were confirmed to have ruptures.
- 87.8% of women whose scans indicated a rupture said they would remove the ruptured implant within 12 months.
- Almost 100% said they would get future ultrasound screenings for silent rupture.
While the study was conducted with clinicians who received only one day of training, Dr. Salzman has never been surprised in the operating room. Through years of experience, Dr. Salzman has developed a point-of-care ultrasound breast scanning protocol for effective implant screening. Dr. Salzman currently uses the Clarius handheld ultrasound scanner every day. Watch his 7-minute interview to learn why he believes ultrasound is essential for every plastic surgeon.
New HD3 Handheld Ultrasound Scanner and Advanced Software for Breast Screening
Thank you, Surgical Times for spreading the news about our 10 new Clarius HD3 ultrasound scanners that are now available across several continents. The Clarius L7 HD3 and Clarius L15 HD3 are both suitable for breast screenings. The Clarius Advanced Breast Package offers streamlined workflow and optimized applications for diagnostic examinations and interventional procedures.
Contact us today or request an ultrasound demo to see how high-definition imaging can help you improve safety and deliver consistent patient outcomes in your plastic surgery practice.