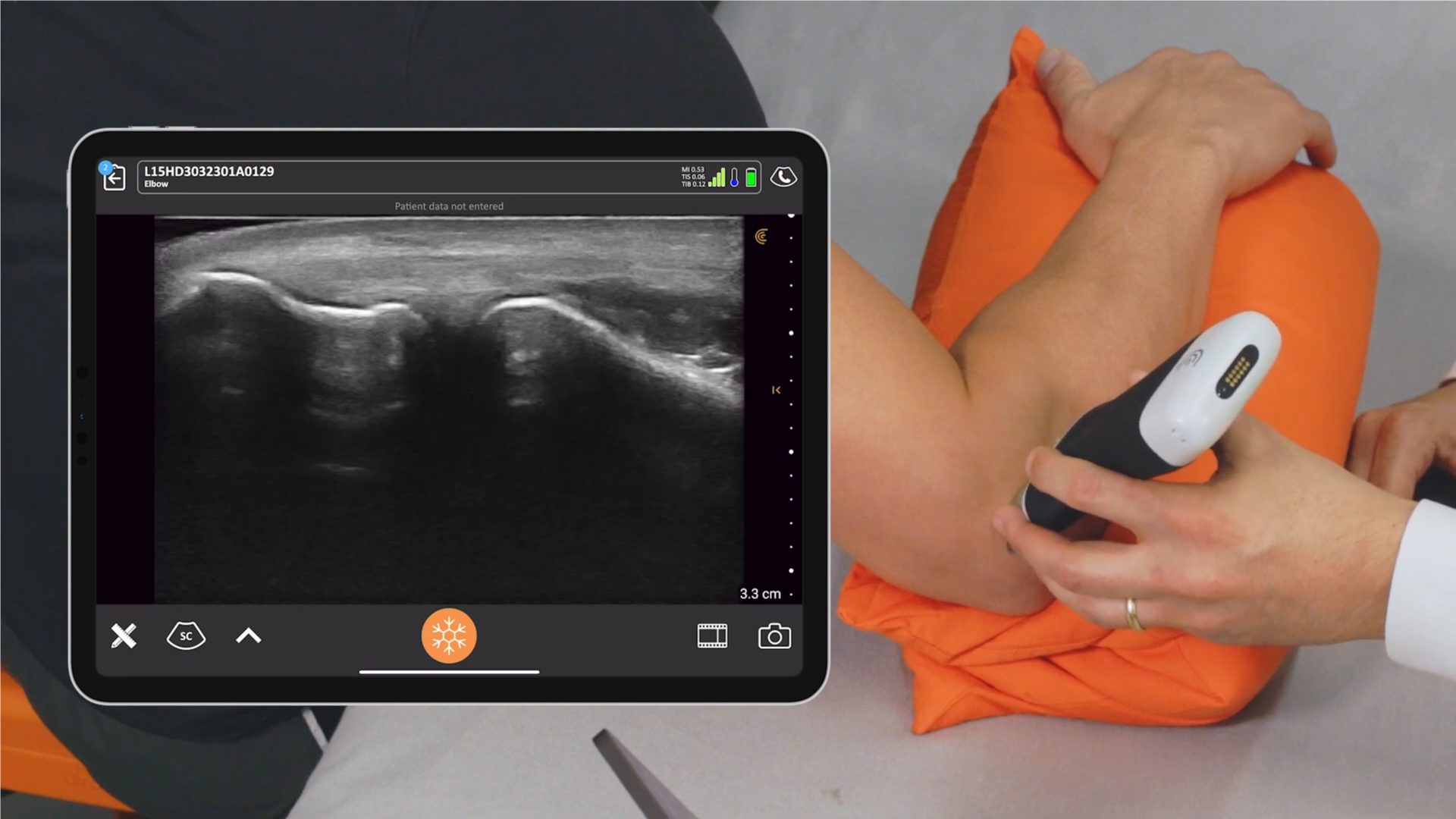
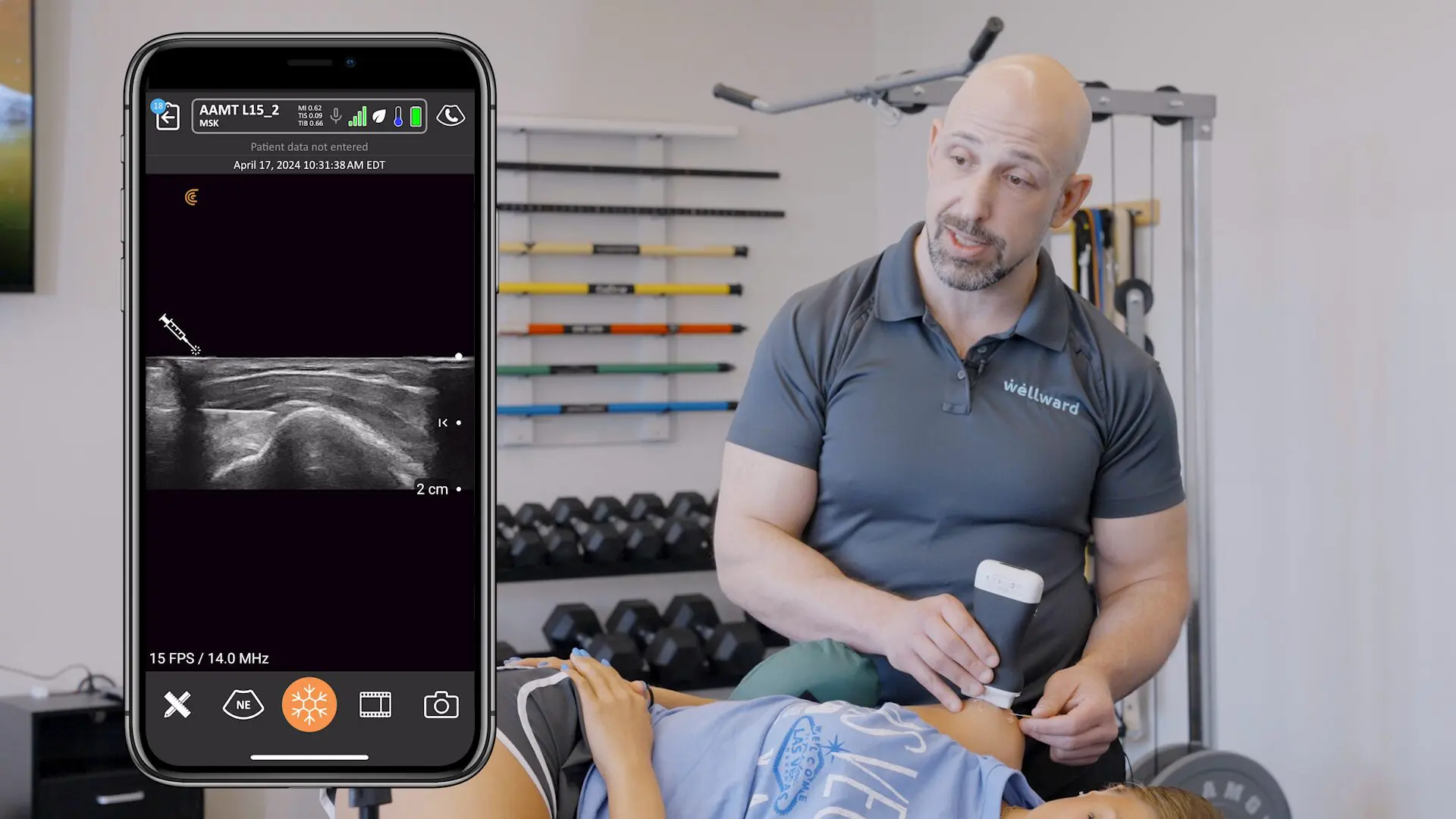
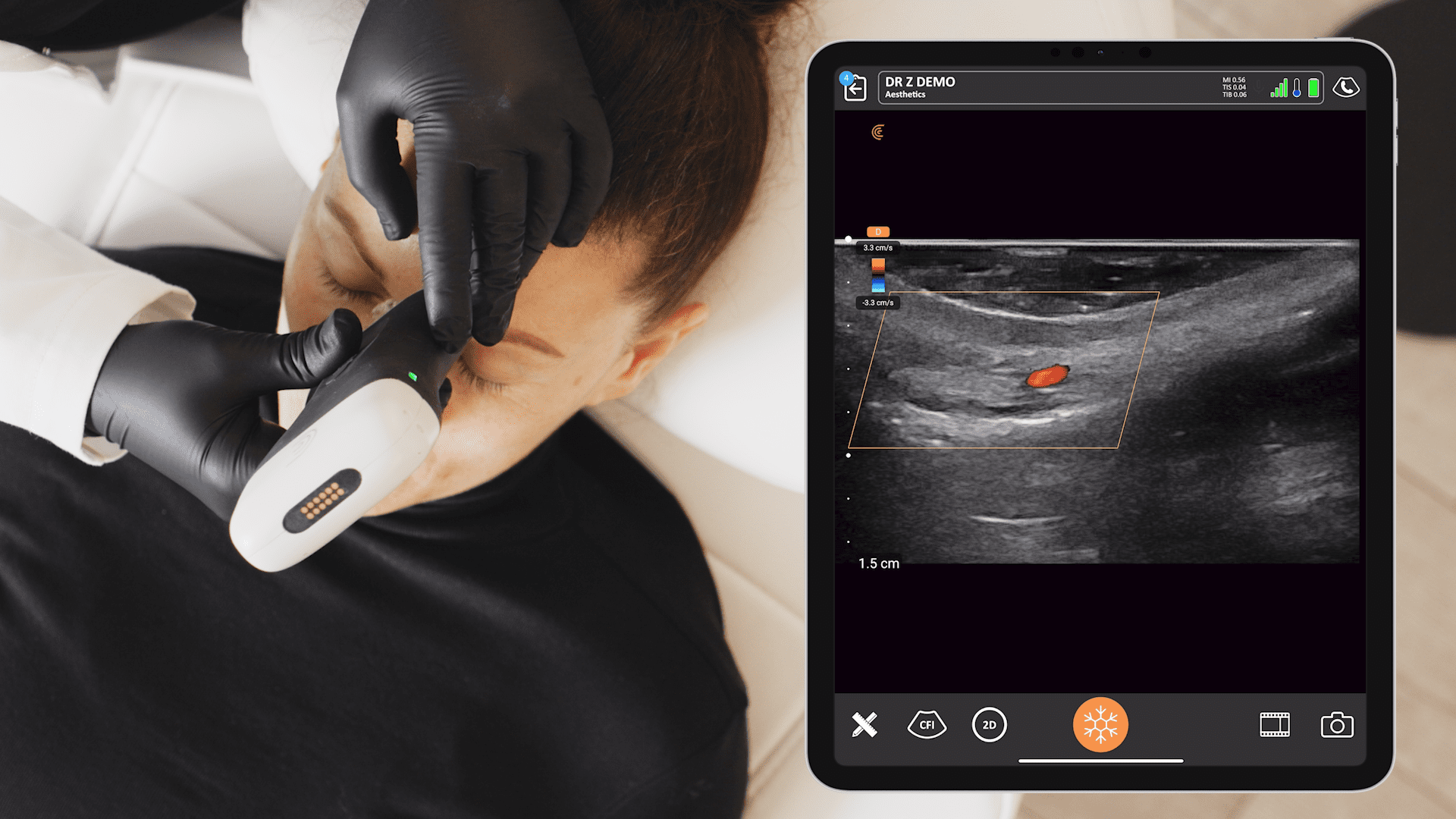
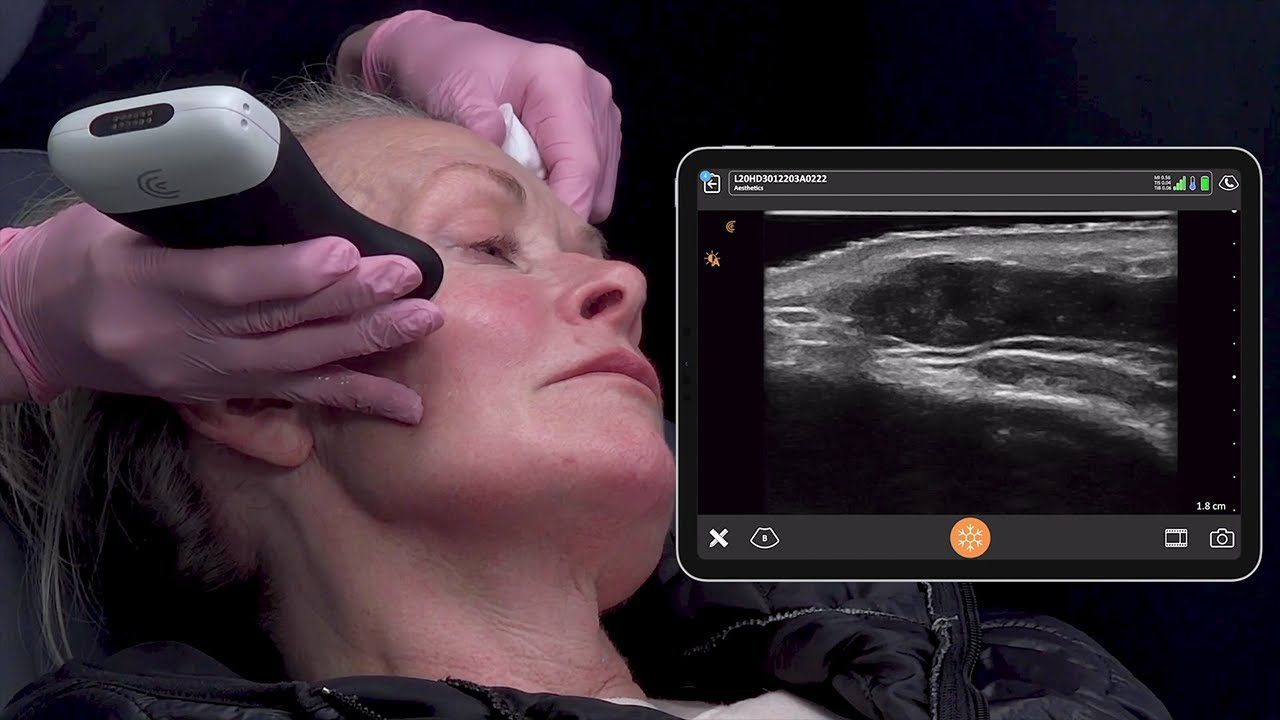
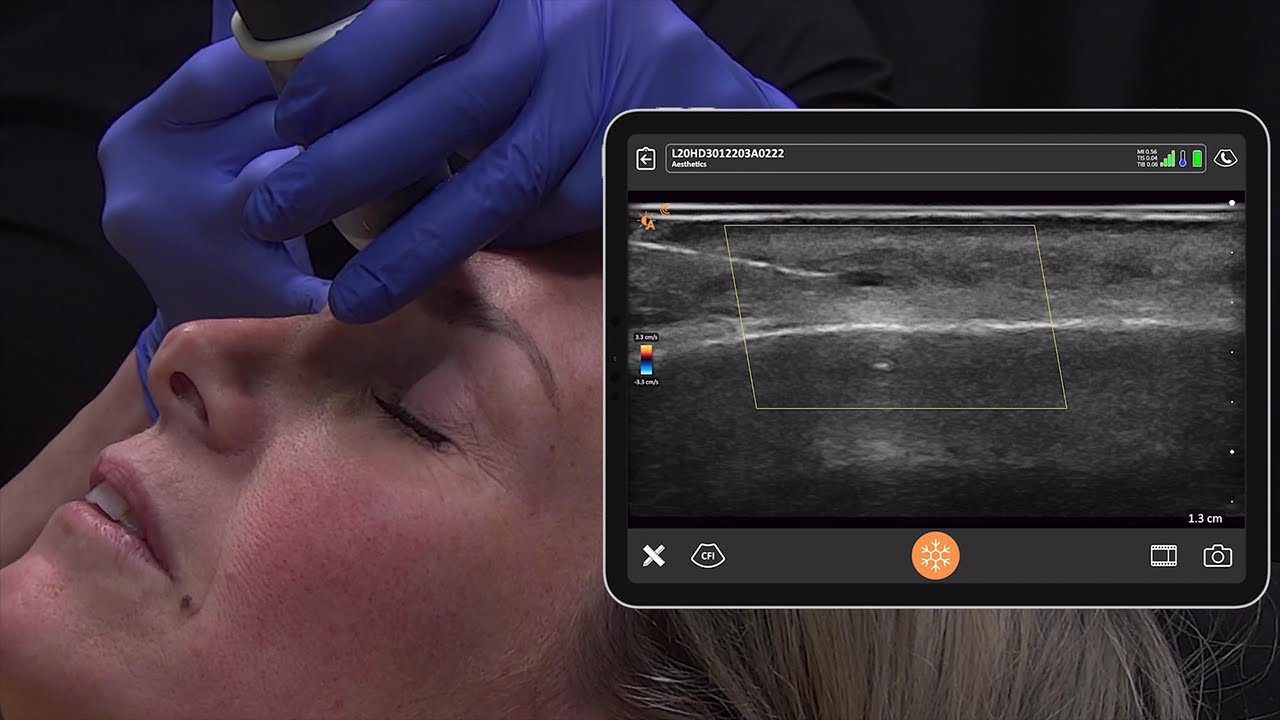
We receive a lot of questions about regulatory clearance. Is Clarius available for purchase in my country? How much longer do I have to wait? Why do I need approval?
To answer these questions and more we sat down with our Abhijit Ahir, our head of Regulatory and Quality Affairs, and he shared some news about the availability of Clarius.
Where is Clarius currently available for sale?
As of January 2018, Clarius Ultrasound Scanners are currently being sold in the following countries/markets:
| Canada | Malta |
| USA | Netherlands |
| New Zealand | Croatia |
| Australia | Cyprus |
| Chile | Estonia |
| UK | Slovenia |
| Portugal | Switzerland |
| Spain | Germany |
| Ireland | Austria |
| Luxembourg | France |
| Italy | Turkey |
| Hong Kong | Colombia |
| Saudi Arabia | Israel |
| Brazil | Taiwan |
| Thailand | *other |
If you don’t see your country on the list, please subscribe to our mailing list and we’ll be in touch as soon as we can sell in your region.
*Other markets where there are no regulatory requirements on the sale of medical devices, or where registration is voluntary.
What approvals/certifications has Clarius received?
Clarius is an ISO 13485-certified company and has received clearances in the above markets. The Clarius Ultrasound Scanner, has gone through rigorous testing towards areas such Electromagnetic Compatibility (EMC), Electrical Safety, Biocompatibility, Bluetooth, Wifi/Wireless/Co-Existence, etc. The product has also been thoroughly validated by ultrasound experts (radiologists, sonographers, etc.) in its intended-use environment and in real-world scenarios.
It has shown superior safety and performance characteristics when compared to its competitors in the various markets and is a game changer in the industry. The fact that the Clarius Ultrasound Scanner is cleared for sale by the largest, the most important, and the most stringent regulatory jurisdictions in the world reflects the rigor and passion with which the product has been built.
What countries/regions is Clarius currently working on regulatory approval?
Clarius is seeking clearance/registration/entry into the following markets:
- Algeria
- China
- Egypt
- Japan
- Kazakhstan
- Mexico
- Russia
- Singapore
- South Korea
- The Ukraine
- Other GCC countries including as Kuwait, The UAE, Qatar, Oman, Bahrain, and Yemen
Why does regulatory approval matter?
A medical device, at a high level, is meant to diagnose or treat a disease or abnormality and cure a human being (also animals in the case of veterinary devices). To the core, medical devices deal with patient health and safety. If these devices are not safe and/or do not perform as intended, human health is at risk, and this may lead to injuries or even death. Governments across the world have an obligation towards good health of their citizens/residents, and this eventually boils down to making sure that the medical devices in circulation in the country are safe and perform as intended; hence the regulatory process of clearing a medical device for sale.
Regulatory jurisdictions are mainly concerned about two things:
Has the medical device been designed and tested for safety and performance?
Regulatory bodies intend to review product documentation/details such as design specifications, test reports, labeling/instructions for use, history of incidents/recalls, etc., to make sure that the product has been designed and verified/validated with safety and performance aspects in mind and that the product functions as intended.
Is the company building the medical device mature enough?
In almost all cases, a manufacturer can demonstrate maturity in key, quality-affecting operations, such as design, manufacturing, complaint processing, human resources, etc., by implementing and getting certified (by a third-party audit firm such as BSI) on an ISO 13485 Quality Management System. ISO 13485 is a framework that focuses specifically on the needs of the medical device industry, and certification on this standard indicates that the company has successfully implemented and maintained stringent processes that are recognized globally and that lead to safe and effective medical devices.
How long does it typically take to achieve such approvals/clearance?
The time it takes to achieve clearance depends on the targeted market. Some markets can clear devices in less than a month, while some can take 12-18 months or beyond. Some markets (e.g., Australia, NZ) accept other country’s clearance (e.g., CE, US 510(k) clearance) to grant market entry, while some markets (e.g., China) require a manufacturer to perform the complete set of already-passed tests again and also possess home-country clearance/approval as part of their clearance process. Different jurisdictions have different processes for clearance, however, most of them are looking for similar product and facility documentation.
It also depends on the risk level of the medical device. The higher the risk, the more rigorous the review process it goes through, thus adding to the timeline. Clarius Ultrasound Scanner is a Class II device (around medium risk) in most jurisdictions, and therefore, the clearance timeline is not as lengthy as that of a device requiring submission of clinical trial data. At the same time, the clearance timeline is also not as quick as that of low risk products such as surgical gloves, ultrasound gel, etc.